Understanding Cortex Side Bonds in Hair: The Science Behind Hair’s Strength and Elasticity
The cortex of the hair strand plays a pivotal role in determining its strength and elasticity. Understanding the cortex side bonds is not just academically fascinating but also practically crucial for cosmetologists and haircare professionals. A thorough comprehension of these bonds can help experts make informed decisions on treatments, styling, and even preventive care for hair damage. This article delves into the science of cortex side bonds and their significance in the realm of cosmetology.

The Anatomy of the Cortex
The cortex is the middle layer of the hair strand, lying between the cuticle and the medulla. It is primarily composed of millions of polypeptide chains. These chains are bound together by side bonds, which function much like the rungs of a ladder.
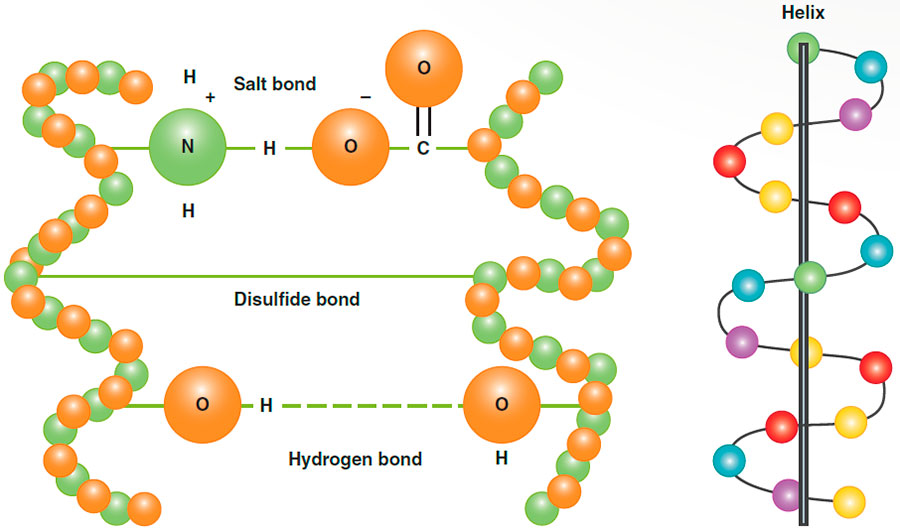
Types of Cortex Side Bonds
Hydrogen Bonds
- Nature: Weak and physical
- Formation: Through the attraction of opposite charges
- Breakage: Easily broken by water or heat, like during wet and thermal styling
- Reformation: When hair dries or cools, these bonds reform. Hence, allowing thermal curls to cool before styling will hold the style longer.
Salt Bonds
- Nature: Weak and physical
- Formation: Like hydrogen bonds, formed through the attraction of opposite charges
- Breakage: Susceptible to changes in pH, broken easily by strong alkaline or acidic solutions
Disulfide Bonds
- Nature: Strong and chemical
- Formation: These bonds link the sulfur atoms of two neighboring cysteine amino acids within the polypeptide chains
- Breakage: Permanent waves and hair relaxers alter these bonds; improper use of these treatments can weaken the hair substantially
Hair Bonds | ||||
Bond | Type | Strength | Broken By | Reformed By |
Hydrogen | Side bond (physical) | Weak | Water or heat | Drying or cooling |
Salt | Side bond (physical) | Weak | Changes in pH | Balancing pH |
Disulfide | Side bond (chemical) | Strong | 1. Thio perms and thio relaxers | 1. Oxidation with neutralizer |
Peptide | End bond (chemical) | Strong | Chemical depilatories | Not reformed; hair dissolves |
Hair Bonds
Practical Applications in Cosmetology
Styling and Treatments
- Curling and Straightening: Knowing that hydrogen bonds are broken and reformed easily helps in achieving the desired shape during thermal styling.
- Chemical Treatments: Understanding the strength of disulfide bonds assists in safely performing procedures like permanent waves or relaxers.
Hair Health
- Damage Prevention: Knowledge of how these bonds break and reform can be instrumental in advising clients on how to avoid activities that would weaken the hair.
- Product Recommendation: Recommending pH-balanced products to preserve salt bonds or protein-rich treatments to strengthen hair.
Research and Product Development
- Creating New Products: Insights into how these bonds work can inform the creation of new hair care products designed to strengthen and protect the hair.
Consultation and Client Education
- Insightful Analysis: A detailed understanding of cortex side bonds allows haircare professionals to offer more insightful consultations, providing advice based on scientific facts rather than mere observation.
Conclusion
Understanding the science of cortex side bonds is vital for any professional in the hair care industry. These bonds, namely hydrogen, salt, and disulfide, are the secret behind hair’s strength and elasticity. Their intricate behavior during different treatments and environmental conditions forms the basis for many services offered in salons. By comprehending the dynamics of these side bonds, cosmetologists can significantly improve the quality of their services, offering personalized, effective, and safe treatments for their clients.







